




FOB Price
Get Latest Price10 ~ 15 USD / ( Negotiable )
|1000 Unit Minimum Order
Country:
China
Model No:
-
FOB Price:
10 ~ 15 USD / ( Negotiable ) Get Latest Price
Place of Origin:
-
Price for Minimum Order:
10
Minimum Order Quantity:
1000 Unit
Packaging Detail:
-
Delivery Time:
-
Supplying Ability:
20000 Unit per Day
Payment Type:
T/T, Western Union
Product Group :
-
Contact Person Sean
Shanghai, Shanghai
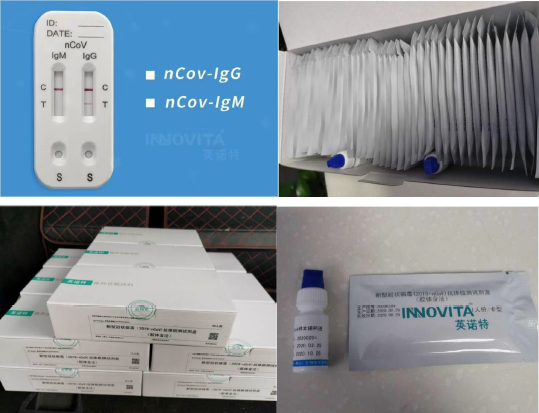
New Coronavirus (****-nCoV) Antibody
Detection Kit (Colloidal Gold)
[Product Name]
Generic Name: New Coronavirus (****-nCoV) Antibody Detection Kit
(Colloidal Gold)
[Drug Packing Specification] *0T/*0T
[Intended Use]
This detection kit is applied to the in-vitro qualitative detection
of IgM/IgG antibody
to new coronavirus (****-nCoV) in human serum, plasma and venous
whole blood
samples. It is only used as a supplementary detection index for the
suspected cases
with negative results of NAT for ****-nCoV, or used in combination
with NAT for the
diagnosis of the suspected cases, and it may not be used as the
basis for the
diagnosis and exclusion standard of COVID**9. It is not applicable
to the screening in
the general population.
It is only used by medical institutions.
It is recommended that the positive detection result should be
further verified and
confirmed, and the negative result may not exclude the possibility
of virus infection.
This product is only used for clinical application and emergency
stockpile during the
epidemic of COVID**9 which broke out in December of ***9. It may
not be used as a
conventional in-vitro diagnosis reagent in clinical application.
The detection result
provided by this kit is only for reference. It is recommended to
make a comprehensive
analysis of the disease in combination with a patients clinical
manifestations and
other laboratory tests.
It is required to perform the laboratory tests for ****-nCoV in
accordance with the
requirements established by the "Technical Guidelines of Laboratory
Tests for
COVID**9 and other specifications in order to guarantee the
biosafety.
[Testing Principle]
This product is used to detect IgM/IgG antibody to ****-nCoV by
means of the immunocapturing-based method. Nitrocellulose
membranes in this product are
coated with mouse anti-human IgM (μ chain) monoclonal antibody,
mouse
anti-human IgG (γ chain) monoclonal antibody and goat anti-mouse
IgG antibody
respectively, with colloidal gold-labeled recombinant ****-nCoV
antigen and mouse
IgG antibody used as tracers. When in use, the sample to be tested
is added into the
loading wells of IgM and IgG antibody detection reagent cards
respectively. If the
sample contains IgM antibodies to ****-nCoV, they will form
compounds by binding
to colloidal gold-labeled ****-nCoV antigens, and these compounds
can be captured
at the coating of mouse anti-human IgM antibodies and bound to them
to form
complexes shown as the purplish red band; if the sample contains
IgG antibodies to
****-nCoV, they will form compounds by binding to colloidal
gold-labeled
****-nCoV antigens, and these compounds can be captured at the
coating of mouse
anti-human IgG (γ chain) monoclonal antibodies and bound to them to
form
complexes shown as the purplish red band. The colloidal
gold-labeled mouse IgG
antibodies bind to goat anti-mouse IgG antibodies and form
complexes shown as the
purplish red band, which is used as the control line (C-line).
[Main Components]
1. Detection Card. The detection card, which is composed of a
plastic plate, NCM,
absorbent paper, whole blood separation membranes, sample pads and
gold
conjugation pads, contains recombinant ****-nCoV antigen, mouse
anti-human IgM
(μ chain) monoclonal antibody, mouse anti-human IgG (γ chain)
monoclonal
antibody, mouse IgG antibody and goat anti-mouse IgG antibody.
2. Sample Diluent: *0 mM PBS, casein, PC**0; 6.0 mL × 1 bottle
(*0T) or 6.0 mL × 2
bottles (*0T).
3. Desiccant.
[Storage Conditions & Validity Period]
This product is required to be preserved under the temperature of
*0℃ ~ *0℃, and
the validity period is provisionally expected to be six months.
It is recommended that the detection card should be used below the
humidity of *0%
within one hour after removing the packing materials.
See the label for the production date and the expiry date.
[Sample Requirements]
It is required to collect serum (plasma) or venous whole blood
samples in a conventional manner.
If the collected venous whole blood sample is placed under the
temperature of 2℃ ~
8℃, it will be preserved for three days. Cryopreservation is
forbidden. The venous
whole blood sample can be prevented from coagulation by using
conventional
dosages of heparin (9.8 - *8 IU/mL), sodium citrate (3.8%,
equivalent to **9 mmol/L)
and ETA (4.*5 mmoL/mL ± 0.*5 mmol/mL).
If the collected serum sample is placed under the temperature of 2℃
~ 8℃, it can be
preserved for seven days. If it is placed under the temperature no
more than **0℃, it
will be provisionally preserved for six months. Avoid repeated
freezing and thawing
(no more than 8 times). It is best to detect the sample on the day
of collection.
It is also important to note that it is required to collect and
preserve samples strictly in
accordance with aseptic procedures.
[Testing Methods]
Open the aluminum foil bag and take out the detection card.
When detecting, *0 μL of venous whole blood or *0 L of serum
(plasma) sample to be
detected is added into the round loading wells of IgM and IgG
antibody detection
reagent cards respectively, and *0 μL (or 2 droplets) of sample
diluent is added
respectively. It is required to place the sample under the room
temperature in order
to observe the result in *5 minutes.
[Testing Result Interpretation]
It is recommended to observe and record the result in *5
minutes.
IgM antibody positivity: if the interpretation window of IgM
antibody detection
reagent shows clear purplish red band both on T line and C line, it
is interpreted to be
positive for IgM antibodies against ****-nCoV.
IgG antibody positivity: if the interpretation window of IgG
antibody detection
reagent shows clear purplish red band both on T line and C line, it
is interpreted to be
positive for IgG antibodies against ****-nCoV.
Antibody negativity: if the interpretation windows of IgM and IgG
antibody show
clear purplish red band only on C line, it is interpreted to be
negative against
****-nCoV.
Void: if the interpretation window shows no purplish red band on C
line, the test is
interpreted to be void no matter whether there is a purplish red
band on T line in the
interpretation window of IgM or IgG antibody. It is required to
make another detection.
Result interpretation time: it is required to interpret the result
in *5 minutes after
adding the sample to be detected into the loading wells. The result
shown after *5
minutes is interpreted to be void.
[Testing Method Limitations]
This product is only applicable to the qualitative detection and
the auxiliary diagnosis;
At the early stage of infection, no generation or low titers of IgM
and IgG antibodies
to ****-nCoV may result in the negative results. It is supposed to
make another
detection in 7 - *4 days. During the reexamination, it is needed to
make a parallel
detection of the sample collected last time in order to identify
whether seroconvesion
or the up-regulated titer exists or not.
With regard to the patients with immunologic function impaired
or
immunosuppressive therapies received, the reference value of
serological antibody
detection tests is limited;
IgM antibody positivity not only occurs in the primary infection,
but also exists in the
secondary infection;
IgG antibody positivity indicates previous infection or secondary
infection have
already been in existence.
To identify the infection of ****-nCoV is required to be in
combination with patients
clinical manifestations and other methods simultaneously.
[Product Performance Indicators]
All products have been tested by use of national or enterprise
reference panel. The
testing results meet the detection requirements of national or
enterprise reference
panel.
The results of the initial concentration of the sample with the
titer of 1:**0 in IgM and
IgG antibodies to ****-nCoV show no hook effects.
The tests of **6 clinically confirmed cases have indicated that the
antibody detection
results of the assessed reagents show that the number of the
positive samples is **0
(Sensitivity: *7.3%, *5% CI: *0.*0% ~ *2.0%); The tests of *2
clinically excluded cases
have indicated that the antibody detection results of the assessed
reagents show that
the number of the negative samples is *2 (Specificity: **0%, *5%
CI: *4.*0% ~ **0%).
Avoid using special samples: hyperlipemia (triglyceride
concentration is over *5
mg/mL), bilirubin (the concentration is over 0.2 mg/mL) and
hemolysis (hemoglobin concentration is over 5.0 mg/mL) serum
samples may result in red background and
other phenomena, affecting the interpretation of the detection
results to some extent.
Therefore, it is recommended to avoid using these samples.
This product shows no cross reaction to HKU*-IgM antibody, OC**-IgM
antibody,
NL**-IgM antibody, **9E-IgM antibody, Influenza A virus H1N1 (***9
A/H1N1
influenza virus, seasonal H1N1 influenza virus) IgM antibody,
H3N*-IgM antibody,
H5N*-IgM antibody, H7N*-IgM antibody, Influenza B virus IgM
antibody, respiratory
syncytial virus IgM antibody, adenovirus IgM antibody, rhinovirus
IgM antibody,
enterovirus A-IgM antibody, EB virus IgM antibody, measles virus
IgM antibody,
cytomegalovirus IgM antibody, RV-IgM antibody, mumps virus IgM
antibody,
VZV-IgM antibody, PIV-IgM antibody, M. Pneumonia IgM antibody,
Cpn-IgM
antibody and coxsackievirus B IgM antibody.
This product shows no cross reaction to HKU*-IgG antibody, OC**-IgG
antibody,
NL**-IgG antibody, **9E-IgG antibody, Influenza A virus H1N1 (***9
A/H1N1
influenza virus, seasonal H1N1 influenza virus) IgG antibody,
H3N*-IgG antibody,
H5N*-IgG antibody, H7N*-IgG antibody, Influenza B virus IgG
antibody, respiratory
syncytial virus IgG antibody, adenovirus IgG antibody, rhinovirus
IgG antibody,
enterovirus A-IgG antibody, EB virus IgG antibody, measles virus
IgG antibody,
cytomegalovirus IgG antibody, RV-IgG antibody, mumps virus IgG
antibody, VZV-IgG
antibody, PIV-IgG antibody, M. Pneumonia IgG antibody, Cpn-IgG
antibody and
coxsackievirus B IgG antibody.
This product shows no cross reaction to RF, ANA and AMA.
Conventional antiviral drugs such as epinastine hydrochloride (≤ 4
mg/L), ribavirin (≤
*0 mg/L), IFN (≤ **0 mg/L), oseltamivir (≤ *0 mg/L), arbidol (≤ *0
mg/L),
levofloxacin (≤ **0 mg/L), azithromycin (≤ **0 mg/L), ceftriaxone
(≤ **0 mg/L) and
meropenem (≤ **0 mg/L) have no interference effect on this product
during the
detection.
No interference effect of systemic lupus erythematosus on the kit
is seen during the
detection.
No interference effect of non-specific IgM antibodies (≤ 0.8 mg/mL)
and
non-specific IgG antibodies (≤ 4 mg/mL) on the kit is seen during
the detection.
Anticoagulants such as heparin, sodium citrate and EDTA have no
interference effects
on the kit during the detection.
Different experimental personnel have performed precision
experiments by using this
kit at different time in different places. The experimental results
meet the
requirements of the product performance.
If the sample with specific IgM antibody positivity is destroyed
by
*-Hydroxy**-ethanethiol, IgM antibody detection will show negative
results.
According to the preliminary assessment, it is basically confirmed
that the clinical
performance of this product can meet the emergency need for the
epidemic. After
product launching, the clinical performance of this product will be
further verified by
collecting more clinical data. The clinical experiments of this
product are conducted
on the basis of disease diagnosis/exclusion standards established
by Diagnosis and
Treatment Scheme for COVID**9. The clinical researches are
performed in 5 medical
institutions. The included cases are suspected cases with
****-nCoV. There are **6
confirmed cases and *2 excluded cases in **7 included cases. The
research results are
shown as follows: clinical sensitivity *7.*0% (*5% CI: *0.*0% ~
*2.0%), specificity
**0% (*5% CI: *4.*0% ~ **0%). The types of samples assessed
clinically cover serum,
plasma and venous whole blood. After product launching, the
clinical performance of
this product will be further verified by collecting more clinical
data.
[Notes]
It is recommended to use fresh samples and avoid using
contaminated, hemolysis,
jaundice or hyperlipemia samples.
The result shown by this kit after *5 minutes is void.
| Country: | China |
| Model No: | - |
| FOB Price: | 10 ~ 15 / ( Negotiable ) Get Latest Price |
| Place of Origin: | - |
| Price for Minimum Order: | 10 |
| Minimum Order Quantity: | 1000 Unit |
| Packaging Detail: | - |
| Delivery Time: | - |
| Supplying Ability: | 20000 Unit per Day |
| Payment Type: | T/T, Western Union |
| Product Group : | - |