
FOB Price
Get Latest Price|
5000 Piece Minimum Order
Country:
China
Model No:
DNSHAV
FOB Price:
Place of Origin:
China
Price for Minimum Order:
-
Minimum Order Quantity:
5000 Piece
Packaging Detail:
1 test and desiccant in a single pouch
Delivery Time:
according to your quantity
Supplying Ability:
750000 Piece per Month
Payment Type:
T/T, Western Union
Product Group :
Contact Person Ms. ellen
Room 506, High-tech Innovation Center, Zhongyang Road,, Nantong, Jiangsu
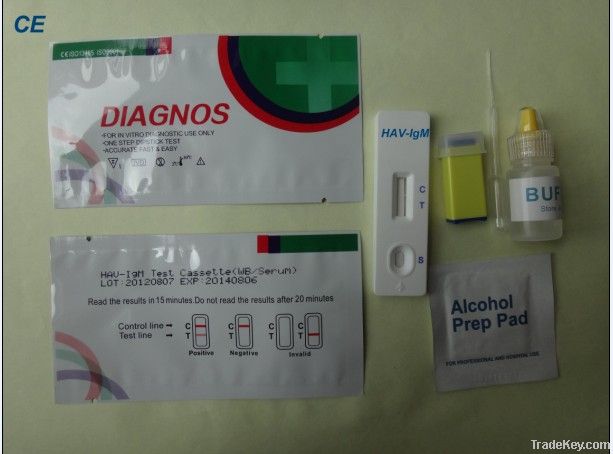
Medical HAV-IGM
rapid Test Device
size: 2.5mm,3mm,3.5mm,4.0mm(strip)/3.0mm,4.0mm(cassette)
Intended Use:
The OnSite HAV IgM Rapid Test is a lateral flow chromatographic immunoassay for thequalitative detection of IgM antibody to Hepatitis A virus (HAV) in human serum or plasma. It isintended to be used as a screening test and as an aid in the diagnosis of infection with HAV.Any reactive specimen with the OnSite HAV IgM Rapid Test must be confirmed with alternativetesting method(s) and clinical findings.
Procedure:
Step 1:
Bring the specimen and test components to room temperature if
refrigerated or frozen. Mix the specimen well prior to assay once
thawed.
Step 2: When ready to test, open the pouch at the notch and
remove device. Place the test device on a clean, flat
surface.
Step 3: Be sure to label the device with specimen’s ID
number.
Step 4: Fill the pipette dropper with the specimen. Holding the
dropper vertically, dispense 1 drop (about ****5 μL) of specimen
into the sample well making sure that there are no air
bubbles.
Then add 1 drop (about ****0 μL) of
Sample Diluent immediately. (for whole blood only)
Step 5:
Set up timer
Step 6: Results can be read in *5 minutes. Positive results can
be visible in as short as 1 minute.
Don’t read result after *5 minutes. To avoid confusion, discard the test device after interpreting the result.
Interpretation of the results:
NEGATIVE RESULT: If only the C band is developed, the test indicates that no detectable IgM anti-HAV is present in the specimen. The result is negative.
POSITIVE RESULT: If both C and T bands are developed, the test indicates for the presence of IgM anti-HAV in the specimen. The result is positive.
INVALID: If no C band is developed, the assay is invalid regardless of color development on the T band as indicated below. Repeat the assay with a new device.
| Country: | China |
| Model No: | DNSHAV |
| FOB Price: | Get Latest Price |
| Place of Origin: | China |
| Price for Minimum Order: | - |
| Minimum Order Quantity: | 5000 Piece |
| Packaging Detail: | 1 test and desiccant in a single pouch |
| Delivery Time: | according to your quantity |
| Supplying Ability: | 750000 Piece per Month |
| Payment Type: | T/T, Western Union |
| Product Group : | Infectious Disease Test |