
FOB Price
Get Latest Price( Negotiable )
|2000 Piece Minimum Order
Country:
China
Model No:
tni1
FOB Price:
( Negotiable ) Get Latest Price
Place of Origin:
China
Price for Minimum Order:
-
Minimum Order Quantity:
2000 Piece
Packaging Detail:
1 test and a desiccant per foil pouch, box, carton.
Delivery Time:
according to your quantity
Supplying Ability:
10000000 Piece per Month
Payment Type:
T/T, Western Union
Product Group :
Contact Person Ms. ellen
Room 506, High-tech Innovation Center, Zhongyang Road,, Nantong, Jiangsu
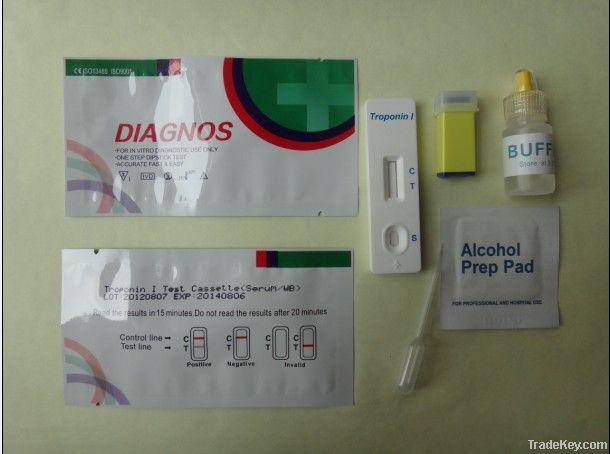
TnI Troponin I Cassette Test
PRINCIPLE:
The
One Step Troponin I Test Device (Whole Blood/Serum/Plasma) is a
qualitative, membrane based immunoassay for the detection of cTnI
in whole blood, serum or plasma. The membrane is pre-coated with
capture reagent on the test line region of the test. During
testing, the whole blood, serum or plasma specimen reacts with
the particle coated with anti-cTnI antibodies. The mixture
migrates upward on the membrane chromatographically by capillary
action to react with capture reagent on the membrane and generate
a colored line. The presence of this colored line in the test
line region indicates a positive result, while its absence
indicates a negative result. To serve as a procedural control, a
colored line will always appear in the control line region
indicating that proper volume of specimen has been added and
membrane wicking has occurred.
PRECAUTIONS:
• For
professional in vitro diagnostic use only. Do not use after
expiration date.
• The test
device should remain in the sealed pouch until use.
• Do not
eat, drink or smoke in the area where the specimens or kits are
handled.
• Do not
use if pouch is damaged.
• Handle
all specimens as if they contain infectious agents. Observe
established precautions against microbiological hazards
throughout the procedure and follow the standard procedures for
proper disposal of specimens.
• Wear
protective clothing such as laboratory coats, disposable gloves
or eye protection when specimens are being tested.
• Humidity
and temperature can adversely affect results.
STORAGE AND STABILITY:
Store as
packaged in the sealed pouch either at room temperature or
refrigerated (***0°C). The test device is stable through the
expiration date printed on the sealed pouch. The test device must
remain in the sealed pouch until use. DO NOT FREEZE. Do not use
beyond the expiration date.
INTERPRETATION OF RESULTS:
POSITIVE:
Two
distinct colored lines appear. One line should be in the control
line region (C) and another line should be in the test line
region (T).
*NOTE:
The intensity of the color in the test line region (T) will vary
depending on the concentration of cTnI present in the specimen.
Therefore, any shade of color in the test line region (T) should
be considered positive.
NEGATIVE:
One colored line appears in the control line region (C). No apparent colored line appears in the test line region (T).
INVALID:
Control line (C) fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
QUALITY CONTROL:
An
internal procedural control is included in the test. A colored
line appearing in the control line region (C) is an internal
procedural control. It confirms sufficient specimen volume,
adequate membrane wicking and correct procedural
technique.Control
standards are not supplied with this kit; however it is
recommended that positive and negative controls be tested as a
good laboratory practice to confirm the test procedure and to
verify proper test performance.
| Country: | China |
| Model No: | tni1 |
| FOB Price: | ( Negotiable ) Get Latest Price |
| Place of Origin: | China |
| Price for Minimum Order: | - |
| Minimum Order Quantity: | 2000 Piece |
| Packaging Detail: | 1 test and a desiccant per foil pouch, box, carton. |
| Delivery Time: | according to your quantity |
| Supplying Ability: | 10000000 Piece per Month |
| Payment Type: | T/T, Western Union |
| Product Group : | Cardiac Marker Test |